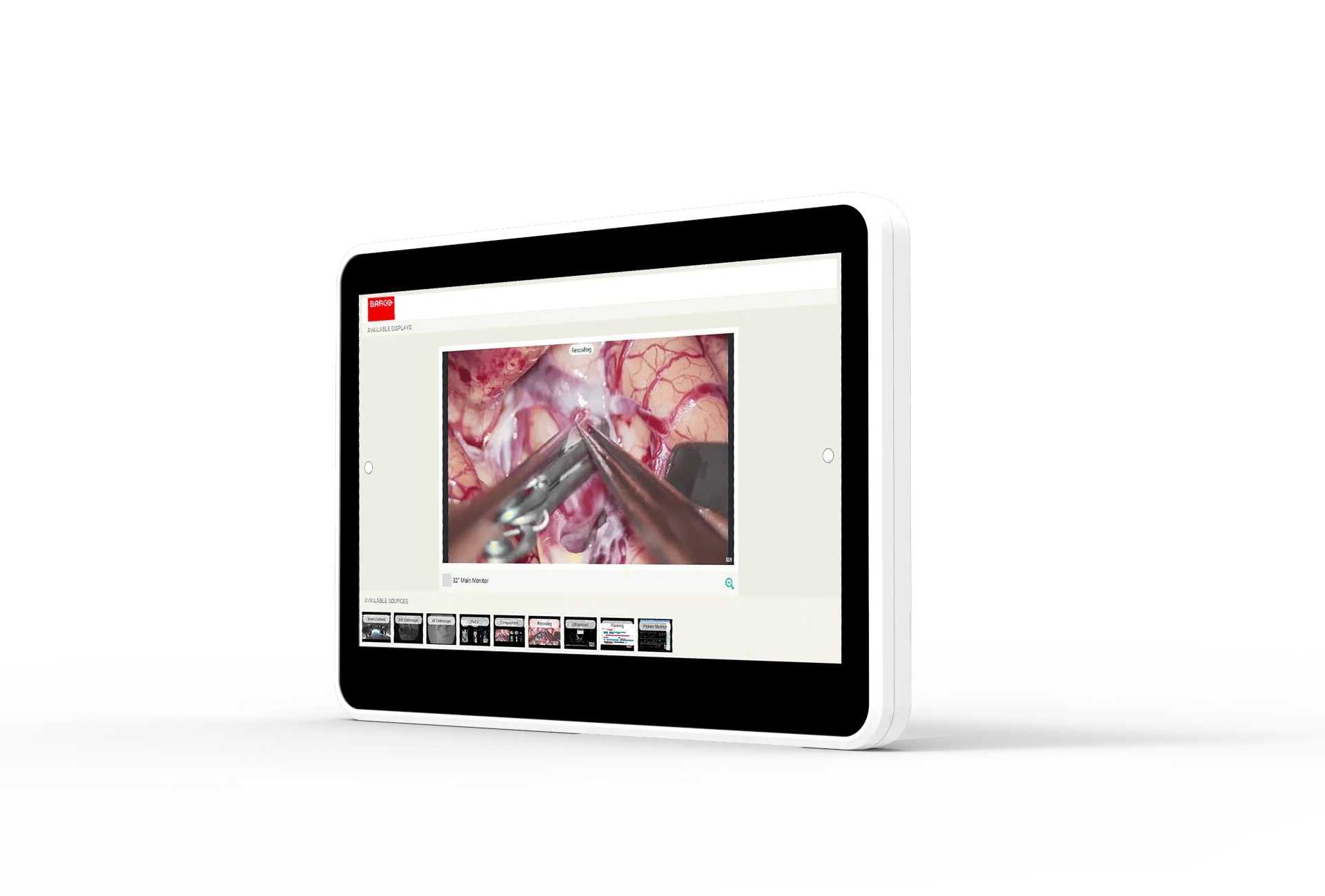
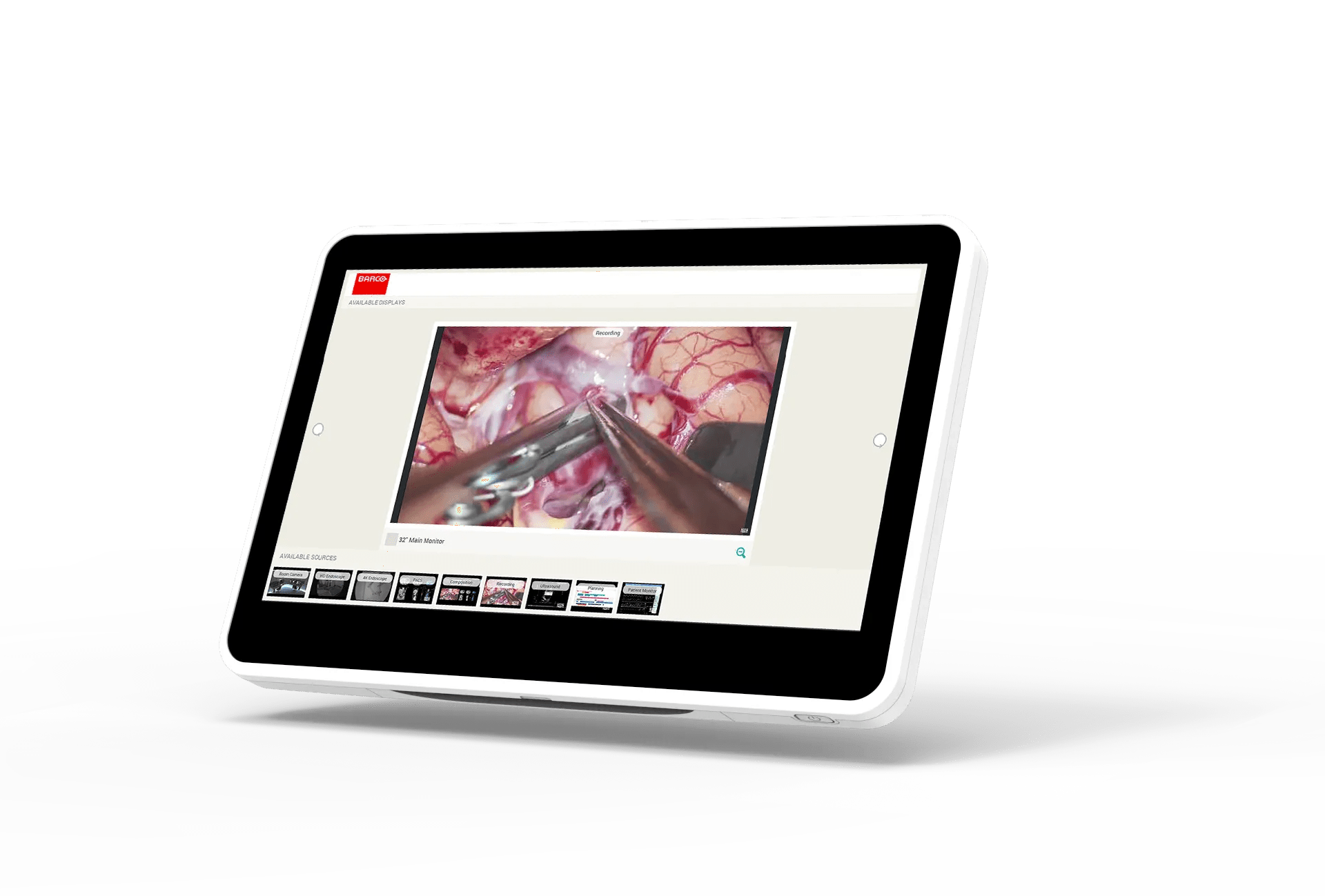
Unlock the potential of your medical workspace with the MUIP-2213, Barco all-in-one user interface device tailored to smarter control in the interventional suite, cathlabs, hybrid OR, or quickly and seamlessly integrated into a wide range of mobile imaging systems, treatment, and robotic surgical devices.
Seamless mobility and point-of-care treatment
- Support the demand for on-the-go applications across the hospital enterprise: it can be used in CathLab, Examination Rooms & Control Rooms, Surgical Rooms and Hybrid Operating Rooms, both inside and outside the patient area.
- Can be used for third party software applications that provide a user interface for medical systems.
- Can be desk, cart, arm, or boom mounted to accommodate a wide range of applications.
Excellent image quality and intuitive touch control
- High-brightness, excellent contrast ratio and its anti-glare front glass makes it ideal for brightly lit clinical environment.
- Easy-to-use with surgical gloves and drapes thanks to its P-CAP Multi-Touch technology.
- Easy-to-see and effortless control due to the MUIP’s combined crisp image quality and wide viewing angle
Medical-Grade ITE Assurance
- Adheres to strictest medical-grade ITE standards and the latest design specifications.
- Sealed fanless thermal design with no ventilation holes eliminates any infection weak point.
- Its IPX3 rating protects against water, surgical fluids and contaminants and allows cleaning according to hospitals infection control guideliness.
Seamless integration and rapid validation
- Engineered according to the most rigorous protocols validation of MUIP-2213 into a medical device or system is quick and streamlined
- Easily connect to any medical device or system via Ethernet or USB cable for hassle-free communication.
Powered by an Intel Pentium N6415 Quad Core CPU, 8 GB RAM, and a 128 GB SSD drive provides high-performance coupled with ultra-low power consumption and multiple connectivity options for a longer useful lifetime.
Configure your MUIP-2213 with the following optional configurations. Alternatively, Barco designs custom solutions from the ground up to deliver the exact performance you need. Minimum order quantities apply.
- Extend the RAM up to 16Gb
- Additional SSD
- Integrated WiFi/BT
- Integrated RFID
Visit https://www.barco.com/en/products/medical-displays/custom-medical-displays
The equipment is not intended to be used for displaying medical images, nor for diagnostic purposes.